Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli
Authors: Alberto Canarini, Christina Kaiser, Andrew Merchant, Andreas Richter and Wolfgang Wanek
Root exudation is an important process determining plant interactions with the soil environment. Many studies have linked this process to soil nutrient mobilization. Yet, it remains unresolved how exudation is controlled and how exactly and under what circumstances plants benefit from exudation. The majority of root exudates including primary metabolites (sugars, amino acids, and organic acids) are believed to be passively lost from the root and used by rhizosphere-dwelling microbes. In this review, we synthetize recent advances in ecology and plant biology to explain and propose mechanisms by which root exudation of primary metabolites is controlled, and what role their exudation plays in plant nutrient acquisition strategies. Specifically, we propose a novel conceptual framework for root exudates. This framework is built upon two main concepts: (1) root exudation of primary metabolites is driven by diffusion, with plants and microbes both modulating concentration gradients and therefore diffusion rates to soil depending on their nutritional status; (2) exuded metabolite concentrations can be sensed at the root tip and signals are translated to modify root architecture. The flux of primary metabolites through root exudation is mostly located at the root tip, where the lack of cell differentiation favors diffusion of metabolites to the soil. We show examples of how the root tip senses concentration changes of exuded metabolites and translates that into signals to modify root growth. Plants can modify the concentration of metabolites either by controlling source/sink processes or by expressing and regulating efflux carriers, therefore challenging the idea of root exudation as a purely unregulated passive process. Through root exudate flux, plants can locally enhance concentrations of many common metabolites, which can serve as sensors and integrators of the plant nutritional status and of the nutrient availability in the surrounding environment. Plant-associated micro-organisms also constitute a strong sink for plant carbon, thereby increasing concentration gradients of metabolites and affecting root exudation. Understanding the mechanisms of and the effects that environmental stimuli have on the magnitude and type of root exudation will ultimately improve our knowledge of processes determining soil CO2 emissions, ecosystem functioning, and how to improve the sustainability of agricultural production.
Introduction
The process of carbon (C) allocation and its adaptability is vitally important for plants to successfully respond to changing environmental conditions. Indeed, maximizing the trade-offs between investments and returns in terms of energy, water, C and nutrients will ultimately determine a plant’s growth, survival, and interaction with its microbiota. External stresses such as competition, nutrient, and/or water limitation cause a series of responses in plants that modify C allocation to maximize the gain of limiting resources. A plethora of research has shown that plant belowground C allocation is tightly connected to water and nutrient cycles (Cheng et al., 2010; McCormack et al., 2015; Gill and Finzi, 2016; Ledo et al., 2018). Yet, a major component of belowground C allocation, namely the process of root exudation, remains poorly understood. It remains unclear why and how plants invest up to 20–40% of their photosynthetically fixed C in root exudates (Badri and Vivanco, 2009). Current ecological theories link root exudation to a benefit for plants via stimulation of beneficial micro-organisms (e.g., symbionts), promoting nutrient acquisition and enabling recognition between self-roots and neighbor-roots (Ortíz-Castro et al., 2009; Dijkstra et al., 2013; Yin et al., 2013; Depuydt, 2014; Mommer et al., 2016; Meier et al., 2017). However, while some root exudates, such as bioactive secondary compounds, are actively exuded from roots through energy-consuming primary or secondary active transporters (Sasse et al., 2018), the majority of them are represented by primary metabolites (mainly sugars, amino acids, and organic acids) in which many studies suggest to be passively lost from the root at the meristematic root apex (McCully and Canny, 1985; Darwent et al., 2003; Jones et al., 2009). In this context, several fundamental questions emerge:1. What is the mechanism driving root exudation of primary metabolites?
2. Do plants have control over this process through adjustments in plant source-sink dynamics and efflux carrier expression and what are the consequences for root growth?
3. Can plants sense the concentrations of exuded and/or soil-borne metabolites?
4. Are these metabolites somehow involved in nutrient foraging through root exudation?
In this manuscript, we propose a conceptual framework built upon recent advances in different disciplines of ecology and biology linking plants with the soil environment. Here, we focus on primary metabolites that are exuded to the soil (sugars, amino acids, and organic acids) and for which specific concentration gradients influence their root exudation. The transient concentrations of these metabolites in the root tip serve as a cue for environmental sensing by plants and signaling between roots and shoots to modify root growth and carbon allocation. Our framework suggests that root exudates are used by the plant to complement the function of nutrient transporters in sensing nutrient availability and in signaling nutrient supply relative to demand. This process therefore optimizes root growth to facilitate effective nutrient foraging and possibly to sense competing neighbors. Also, given that a vast proportion of root exudation is driven by diffusion, soil micro-organisms will play an important role in driving concentration gradients outside the root tip, thus affecting exudation rates. We will utilize examples from studies on root exudation and plant nutrient acquisition strategies to support our framework and then analyze ecosystem scale impacts to highlight the relevance of the proposed mechanism in contributing to soil organic matter decomposition and CO2 emissions, plant community assemblage processes, and plant productivity.
Facilitated Diffusion-Driven Root Exudation and the Role of Micro-Organisms
The majority of root exudation is localized at the root tip (McCully and Canny, 1985; Jaeger et al., 1999; Doan et al., 2017; Sasse et al., 2018). The root tip is the first plant part to explore new soil environment and plays a crucial role in root responses to environmental stimuli. We will now illustrate how primary metabolites can be released from the root tip and how microbes interact with this process. While this manuscript will cover primary metabolites, it is important to highlight that roots also exude a wide range of secondary metabolites and further release high molecular weight compounds into the soil through rhizodeposition, most importantly root border cells and mucilage. These types of rhizodeposits can serve important functions in the soil. For example, they represent an important nutrient source for rhizosphere microbes and influence root-microbe and root-symbiont relations (for example, Hawes et al., 2002, 2016; el Zahar Haichar et al., 2014; Ahmed et al., 2018).Release of Primary Metabolites at the Root Tip
Root exudation is a complex phenomenon encompassing processes that drive C transport to roots and exudation from roots to soil. The long distance transport of C produced in source organs takes place in the phloem, through the widely accepted Munch’s pressure-driven mechanism of phloem flow (Münch, 1930). According to this mechanism, phloem metabolites are transported by a difference in turgor between sink and source organs generated by concentration gradients, which are determined by source-sink activities (De Schepper et al., 2013). Recent experiments support this hypothesis (Knoblauch et al., 2016) and have shed light on how metabolites are unloaded from the phloem to the actively growing root tip (Ross-Elliott et al., 2017), knowledge which is essential to understand root exudation. Specifically, Ross-Elliott et al. (2017) showed that in Arabidopsis, phloem unloading occurs through plasmodesmata in a convective way (combination of mass flow and diffusion). During unloading, low-molecular-weight solutes and proteins are diverted into the phloem-pole pericycle, a tissue connected to the protophloem by a unique class of “funnel plasmodesmata” (Figure 1). While proteins are released in discrete pulses (referred to as “batch unloading”) and remain restricted to the phloem-pole pericycle, low-molecular-weight organic solutes are unloaded without restrictions and move out of the phloem-pole pericycle. The discovery made by Ross-Elliott et al. (2017) is very important in connection to root exudation at the root tip. Indeed, they demonstrate that this area is the principal route for all solutes to be unloaded and that they will move toward the surrounding cells through diffusion (Ross-Elliott et al., 2017) because of the high degree of plasmodesmatal connections in this area (Figure 1; Rutschow et al., 2011).FIGURE 1
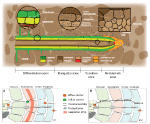
Figure 1. Root structure and areas of root exudation. The upper figure represents the longitudinal section of a root. Tissues are indicated in different colors for the different zones of the root (listed at the bottom). The two circles focus on two distinct zones, a differentiated vs. an undifferentiated area, to show the presence of a Casparian strip and low abundance of plasmodesmata in the differentiated area (left circle), and the presence of funnel plasmodesmata in the undifferentiated area (right circle). The square represents a cross section close to the meristematic area where root exudation is the highest. The lower figures represent a schematic representation of solute movement sites from phloem unloading to the soil environment, either in the differentiation zone (A) or in the undifferentiated root tip (B). (A) Solutes move both through the symplastic and apoplastic pathways, but then they are re-uptaken into the cytoplasm as the Casparian strip limits the apoplastic pathway. Only the cortex and epidermis are responsible for the flux of metabolites into the apoplast and consecutively into the soil (root exudation). Cortex and epidermis represents the major control point for root exudation. (B) At the phloem unloading site, both symplastic and apoplastic pathways are used. Because of the lack of a Casparian strip solutes can move out of the root (root exudation) through both the apoplastic and the symplastic pathway.
Movement of Primary Metabolites Outside the Root Tip
While metabolites can move quite freely through the symplastic pathway, in order to be excreted to the soil environment, they need to pass through at least one plasma membrane to reach the apoplast. The plasma membrane is permeable to gas and to small molecules (such as urea or glycerol), while it is virtually impermeable against larger, uncharged polar molecules (e.g., glucose) and against all charged molecules including ions (Yang and Hinner, 2015). Therefore, these molecules only transit the plasma membranes through specific transmembrane proteins, which form small pores through the lipid bilayer, allowing polar or charged molecules to cross the membrane without interacting with the hydrophobic fatty acid chains of the membrane phospholipids (Sasse et al., 2018).It was recently discovered that the efflux of sugars, organic acids, and amino acids is mediated through specific efflux carriers and channels that may allow a fine tuning of the exudation flux through up/downregulation of their gene expression or at the level of post-translational modification (Badri et al., 2008). Some of these efflux transporters of primary metabolites have been characterized, e.g., for amino acids [UMAMIT transporters (Okumoto and Pilot, 2011; Moe, 2013; Besnard et al., 2016; Dinkeloo et al., 2017), CAT transporters (Yang et al., 2010), GDU transporters (Pratelli et al., 2010)], sugars [probably belonging to the SWEET transporter family (Williams et al., 2000; Chen et al., 2015; Manck-Götzenberger and Requena, 2016)], and organic acids [ALMT/malate and MATE/citrate transporters (Meyer et al., 2010; Mora-Macías et al., 2017)]. Most of these transporters are not primary or secondary active and therefore are not directly coupled to ATP hydrolysis or to ATP-dependent H+ pumps and H+ antiports (see Figure 2). The exception to this is the ATP-dependent ABC transporters for secondary compounds (Badri et al., 2009) and MATE/citrate transporters that possess H+-coupled antiport activity (Meyer et al., 2010). All other transporters were shown not to be coupled to ATP hydrolysis or to H+ cotransport. The common denominator of most of the primary metabolite transporters is that they are substrate-specific facilitators, facilitating the diffusion of primary metabolites across the plasma membrane via transmembrane carriers along the concentration gradient, i.e., from high intracellular to low extracellular concentrations. The exact genes governing the synthesis and abundance of the UMAMIT and SWEET transporters involved in root amino acid and sugar exudation have not yet been identified, and relatively little is currently known regarding the plant demand for nutrients and environmental and edaphic factors that regulate their gene transcription. Only recently, pathogen-driven activation of SWEET expression was shown to increase glucose efflux into the root apoplast (Chen et al., 2015), and Al3+ toxicity or P deficiency triggers exudation of malate by ALMT upregulation in roots (Ma et al., 2001; Kochian et al., 2005; Mora-Macias et al., 2017). Identifying the responsible efflux carriers for the most abundant primary metabolite components of the exudates at the root-soil interface will revolutionize our understanding of the regulation of root exudation (Sasse et al., 2018).
FIGURE 2
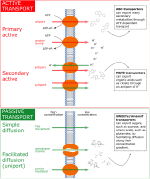
Figure 2. Summary of the main exudation mechanisms through the plasmamembrane at the root tip. The top panel represents active transport mechanisms, either primary active (direct consumption of ATP) or secondary active (e.g. coupled to H+ pumps that actively consume ATP). The bottom panel represents passive transport mechanisms, which allow diffusion following electrochemical gradients. Red arrows represent movement of solutes against their electrochemical gradient, while green arrows represent movement following their electrochemical gradient. On the right side of the figure, examples of membrane transporters allowing movement of primary metabolites are provided.
Once metabolites have moved out of the phloem cell plasma membrane, and they may follow the apoplastic transport pathway. Indeed, a recent study suggests that in immature wheat roots, carbon containing compounds would circulate from the stele to the cortex through an apoplastic pathway and then be used by microbes in the soil environment (Vidal et al., 2018). In the apoplast, the diffusion process would regulate the flux from roots to the soil environment, without any obstacle represented by plasma membranes. This could be very important at the root tip. Indeed, while at distance from the meristem, in the differentiation zone, endodermis cells develop a barrier against apoplastic diffusion (Naseer et al., 2012; Somssich et al., 2016), the meristem lacks an apoplastic barrier (“Casparian strip”; Figure 1). Also, epidermis cell walls in the mature area of root tip of Arabidopsis showed more than 10 times slower apoplastic diffusion rates as compared to the elongation zone (Kramer et al., 2007). It would therefore be interesting to investigate the presence of a root hot spot, where carbon containing compounds are released to the apoplast (Figure 1). If indeed a hot spot could be identified close to the area in which phloem unloading occurs, this would indicate that the apoplastic pathway is a major contributor to root exudation and establishes a close link between the phloem and the soil environment. Indications of an apoplastic pathway are provided by studies showing that bacteria mostly distribute along the axial grooves in between neighboring root epidermal cells (Watt et al., 2006; Schmidt et al., 2018). However, these results could not exclude a direct loss from epidermal cells (Jones et al., 2009) or could indicate that grooves simply represent areas of physical protection for micro-organisms (Schmidt et al., 2018). Indeed, the specific pathway by which solutes move from the unloading area to the external medium is still matter of debate, and a switch from symplastic to apoplastic pathways with root development was also shown (Godt and Roitsch, 2006). More studies on the spatial and temporal dynamics of solute fluxes at the root tip are therefore necessary to improve our understanding of the plant control over the root exudation process.
From reasons outlined above, we therefore postulate that the anatomy of the root tip promotes the diffusion of metabolites (or offer less resistance) from the root apex to the external soil environment and thereby ultimately determines the concentration gradient-dependent outward flux of solutes, i.e., the rate of root exudation. Root tip exudation will be highly dependent on diffusion rates and therefore on concentration gradients between rhizodermal cells and the soil environment. This process will ultimately draw metabolites from the phloem and indicate a close coupling between photosynthetic activity and root exudation, which is strongly influenced by the consumption and transformation of metabolites by microbes in the rhizosphere. This hypothesis is supported by empirical studies showing: (1) localized exudation at the root tip level (McCully and Canny, 1985; Jaeger et al., 1999; Doan et al., 2017); (2) correlation between exudates and root growth (Lucas García et al., 2001); (3) coupling of exudation and photosynthesis (Kuzyakov et al., 2003; Mencuccini and Hölttä, 2010); and (4) reduction of root exudation when plants are allocating resources to reproductive organs instead of root growth (Prikryl and Vancura, 1980; Badri and Vivanco, 2009).
However, it is important to acknowledge two facts. First, many compounds are also exuded by active transport against the concentration gradient (citrate and secondary compounds). Second, while facilitated diffusion-driven root exudation is “passive” in its nature following the concentration gradient from high cytoplasmic to low external concentrations in the soil solution (because diffusion does not require plants to spend energy), plants can still control this process in different ways:
1. Induction/repression of gene expression and post-translational modification of efflux carriers for sugars, amino acids, and organic acids;
2. Possible re-uptake of exuded organic solutes and inorganic nutrients through uptake transporters and their regulation as in (1);
3. Changes in source/sink dynamics including processes at phloem loading sites, import of solutes into root cells and compartmentalization within root epidermal cells (vacuolar loading/sequestration of solutes), and changes in meristematic activity (e.g., root meristem exhaustion).
The above-mentioned mechanisms come at an energy cost for the plant, through secondary active re-uptake of metabolites and by energy-intensive biosynthesis of the efflux and influx carrier proteins. Therefore, we suggest to avoid the use of “passive exudation” to define the root exudation process.





