I seem to remember agrii pushing a similar product a few years ago. I remember my agrii man looking at my soil samples and saying that although my indices were 5 most was locked up(which it probably is on ph 7+ soil) and if I sprayed X product at only Y £££ ha it would release huge amounts of P.
It came in 5lt candle from memory which made me very sceptical to start with, needless to say I didnt bother trying it.
Remind me, have you done FACTS yet? Your Agrii man had obviously conveniently forgotten theirs...
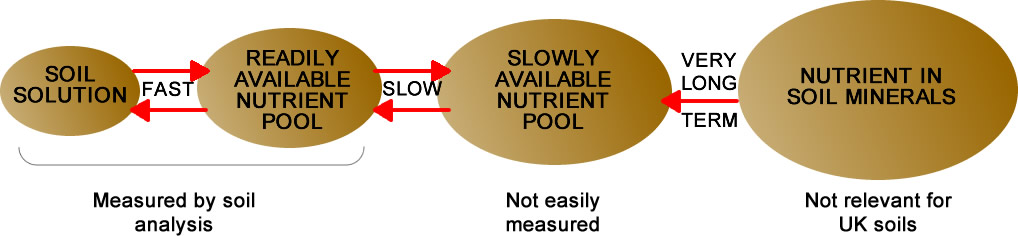
The Olsen P should be crop available but is only the best lab test for most English soils. More peaty soils need a Morgan’s test instead. Of course peak demand may not be supplied sufficiently and there are some TAG results showing yield responses to “fresh” P and K doses even on high index soils.